Contents
What are the challenges of temperature monitoring in pharmaceutical logistics ?
The storage temperature of medicines to be kept cold is 5°C ±3 (or +2/+8°C).
For products to be stored at room temperature, the storage temperature must be between +15 and +25°C.
The transport and storage of medicinal products is governed by the The European GDP Association. For veterinary products, the requirements are similar.
- Plug and Track designs Plug and Play products, i.e. ready to use.
- We attach great importance to providing you with fast decision-making tools.
- So you can concentrate on the job you love, and meet your regulatory obligations with complete peace of mind.
Temperature and environmental control of storage rooms
The control of the storage temperature requires the mapping of the warehouse or cold room. The aim is to determine the hot and cold spots that will need to be monitored.
Mapping should be done in summer and winter to measure the impact of extreme seasons. Recorders used depends on the size and area to be mapped. Nine measurement points are used for a volume of less than 2 m3. For a volume between 2 and 30 m3 , 15 loggers are used. Beyond that the number and location of the temperature sensors must be agreed between you and the expert in auditing.
Standard FDX15-140 or CEI 60068-3-5 serve as a regulatory basis for the placement of recorders, the calculation of indicators (stability, homogeneity, etc.) and the calculation of uncertainties.
Temperature control during the transport of medicines
Good Distribution Practice (GDP) states that it is the distributor’s responsibility to ensure that temperature conditions are kept within acceptable limits. This applies to health products to be stored between +2°C/+8°C, such as those in the +15°C/+25°C range.
How to control temperature in pharmaceutical logistics ?
On site mapping
We offer two solutions for the mapping of climatic chambers :
- The purchase of a complete mapping kit : it includes 9 Thermo Button dataloggers, their calibration certificate, the Thermotrack PC software with mapping module. This module allows you to calculate all the indicators of the FDX 15-140 standard and to create an automatic report.
- The provision of the equipment for the time of the mapping, the study of the positioning of the loggers by one of our experts, the realization of the mapping on site or the sending of the Thermo Buttons. We then provide a complete mapping report with its analysis.
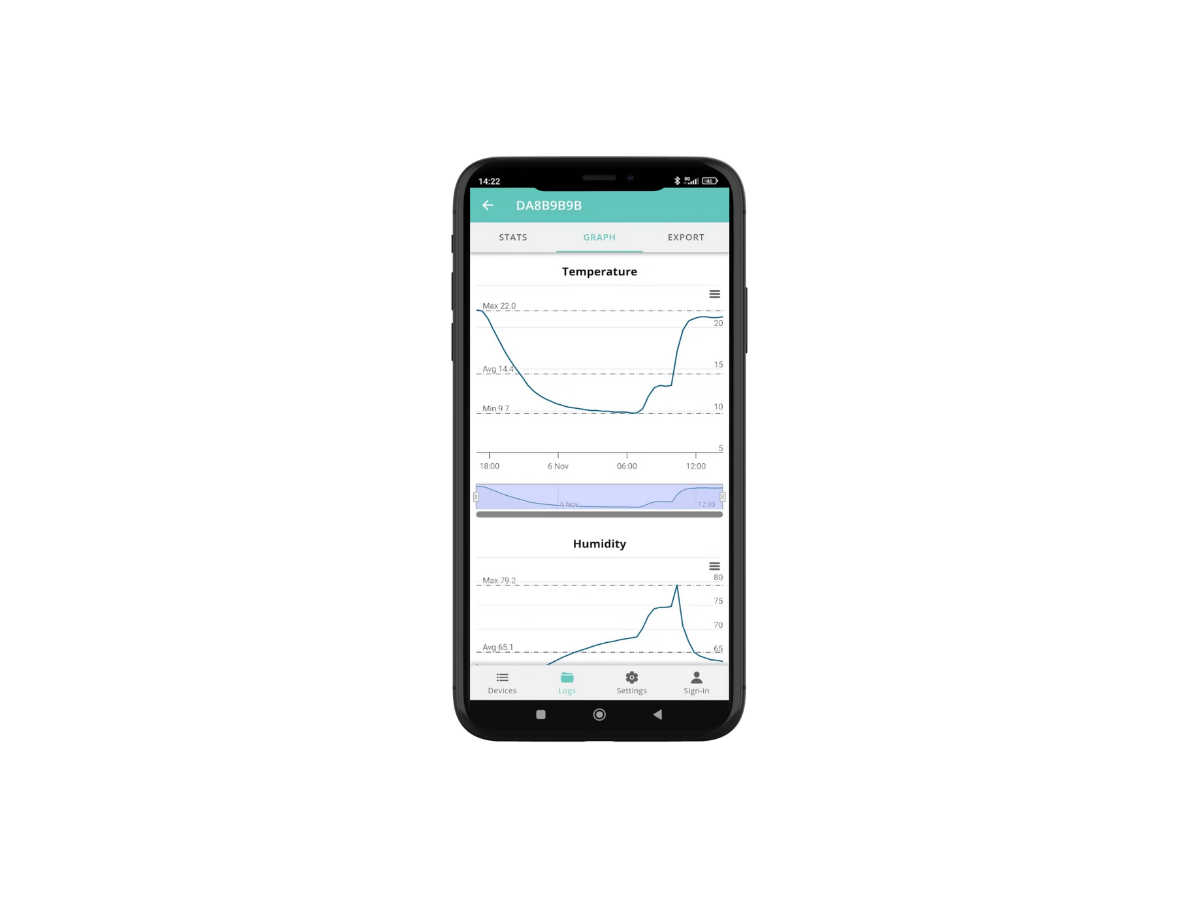
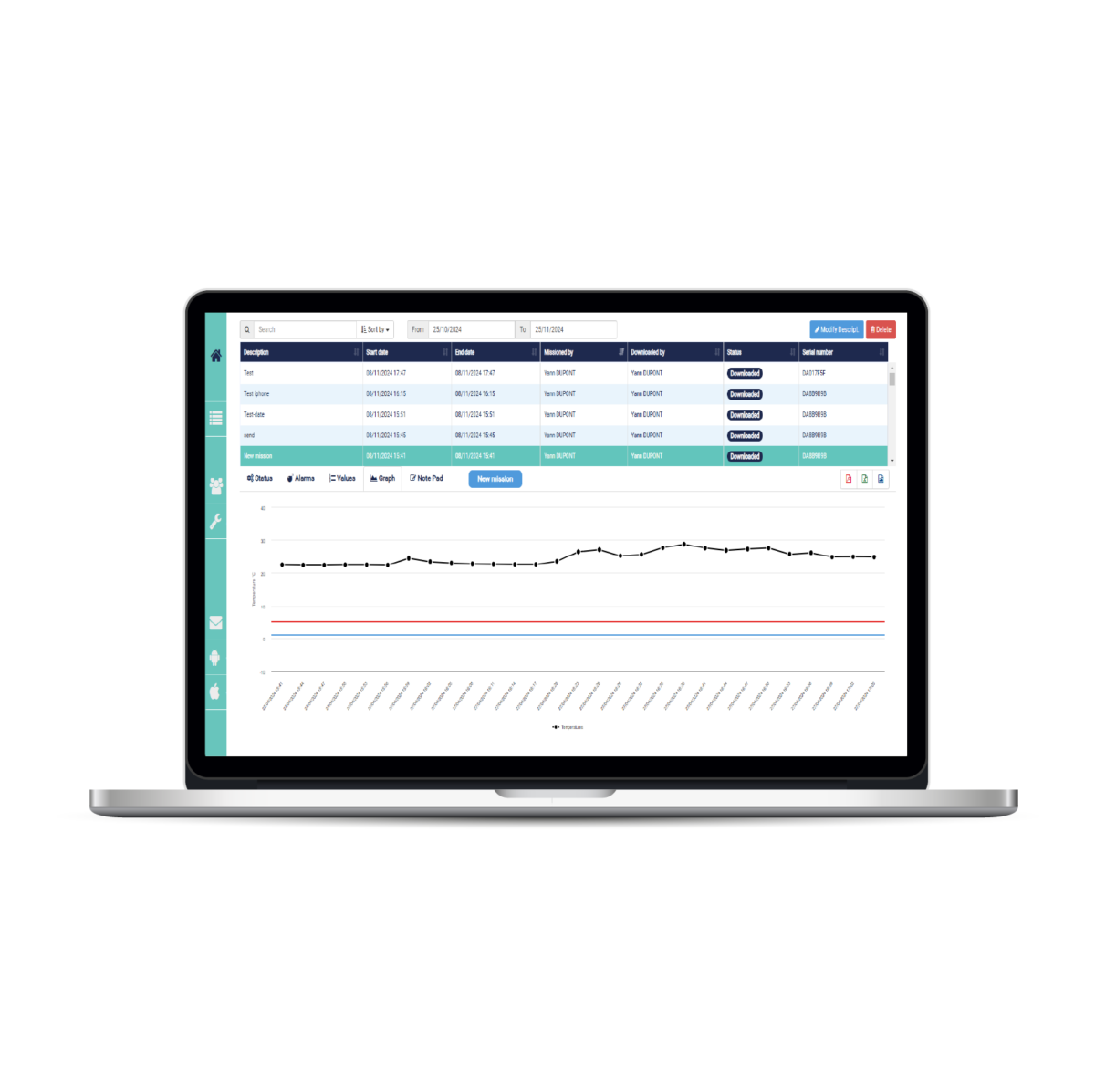
Continuous monitoring
Pour le contrôle de la température et de l’humidité en temps réel, nous vous proposons des sondes de température sans fil FoxNet, ou connexion Wi-Fi ou Ethernet avec Sensor Net Connect, et notre plateforme de surveillance à distance et d’alarme par SMS et Email Thermotrack Webserve, parfaitement adaptée aux exigences de la 21 CFR part 11.
For real-time temperature and humidity monitoring, we offer FoxNet wireless temperature sensors, or Wi-Fi or Ethernet connection with Sensor Net Connect, and our remote monitoring and alarm platform via SMS and Email Thermotrack Webserve, perfectly adapted to the requirements of CFR 21 part 11.
Monitoring of transport temperatures
For temperature control during transport, you can also use the Thermo Buttons 22L (-40/+85°C ±0.5°C). Reusable, with a battery lifespan of 10 years, you will be able to control the temperature from end to end. For the software you can choose between Thermotrack PC or Thermotrack Online, on the Cloud, easier for data sharing between senders and recipients.
Our added value
Thanks to our experience, we can advise you the solution that meets your needs. We have accompanied many pharmacists responsible for logistics or pharmaceutical groups.
We also provide or assist you in the Installation Qualification (IQ) and Operation Qualification (OQ) necessary for the computer validation of your temperature monitoring system.